| PHYSICAL PROPERTIES | |
| Tensile strength | 2.60 N/mm² |
Notched Impact Strength |
2.0 - 45 Kj/m² |
Thermal Coefficient of expansion |
80 x 10-6 |
| Max Cont Use Temp | 60 oC |
| Density | 1.38 g/cm3 |
| RESISTANCE TO CHEMICALS | |
| Dilute Acid | Very good |
| Dilute Alkalis | Very good |
| Oils and greases | Good (variable) |
| Aliphatic Hydrocarbons | Very good |
| Aromatic Hydrocarbons | Poor |
| Halogenated Hydrocarbons | Moderate (variable) |
| Alcohols | Good (variable) |
What is Polyvinyl Chloride (PVC)?
|
| Additives | Properties achieved |
| Anti-oxidants & other stabilizers | Slow down the rate at which the polymer will be degraded by oxygen, heat, visible light or UV radiation |
| Compatibilizers | Enable PVC to be mixed with other plastics and helps plastic recycling |
| Flame retardants | Reduce flammability of plastic |
| Pigments | To colour the plastic |
|
Plasticisers |
To produce flexible and manageable plastic |
| Impact modifiers | To absorb shock without damage |
| Fillers | Inexpensive, inert materials that simply add bulk to the plastic |
Characteristics of Polyvinyl Chloride
These are some of the
properties that makes PVC appropriate for several applications:
- Toughness, strength.
-
Ease of blending, ease of processing
- Flame resistant and fire prevention
properties
For example PVC is difficult to ignite and in the absence of a
powerful external flame will not continue to burn. This is due to its chlorine
compound. This makes it an ideal construction and cable material.
- It is compatible with other additives that can provide PVC clear or
colored, rigid or flexible, etc..
- Excellent electrical insulation
properties. This makes it ideal to be use in cables.
- Impact strength and
resistant to bad weather conditions (i.e. it does not corrode and is  very durable),
appropriate to be used as a construction material
very durable),
appropriate to be used as a construction material
- Resistance to grease, oil
and chemicals
- PVC is chemically stable and does not de-polymerize
-
Density: 1.32-1.42 g/cc
Environmental impact and occupational health and safety aspects of PVC
Manufacture of PVC
The manufacturing plastics often creates
large quantities of toxic chemical pollutants such as dioxin, hydrochloric acid,
and vinyl chloride.
This poses a severe health risks to humans during the
PVC life cycle. These toxins can produce sever illness like cancer, diabetes,
neurological damage, reproductive and birth defects. 
Dioxin is a
persistent Organic Pollutants (POPs), this are chemical substances that persist
in the environment, bio-accumulate through the food chain, and pose a risk of
causing adverse effects to human health and the environment.
In addition, the chloro-ethene monomer is also a carcinogen released during PVC manufacture. This un-reacted monomer can also be present in the final PVC and released during its life cycle.
Plasticizers added to make PVC flexible, may leach out (ex. group phthalates) which are also toxic.
Disposal Plastic was almost too good, as it was durable and degraded very
slowly. On the other hand this same properties is what makes plastic a dangerous
material. Due to the quantity and different additives added to PVC (the PVC
product may consist up to 60% of additives) and also due to its chlorine
contain, the final disposal or recycling of PVC is a issue to be closely
examine.
Plastic was almost too good, as it was durable and degraded very
slowly. On the other hand this same properties is what makes plastic a dangerous
material. Due to the quantity and different additives added to PVC (the PVC
product may consist up to 60% of additives) and also due to its chlorine
contain, the final disposal or recycling of PVC is a issue to be closely
examine.
The options for disposal are recycling, landfill or incineration:
- Recycling
Thermoplastics can be remelted
and reused, although the purity of the material tends to degrade with each reuse
cycle. Furthermore, the separation of the different additives and compounds
forming the plastic makes recycle a difficult option.
The biggest
problem with plastics recycling is that it is difficult to automate the sorting
of plastic waste, and so it is labor-intensive (ex. a mobile might have many
different spare parts made out of different plastic materials).
Thus
because the value of the material is low, recycling plastics is
unprofitable.
Products such as automobiles are now being designed to
make recycling of their large plastic parts easier.
The international
standard for defining environmental claims on products or packaging can be found
in ISO 14021: Environmental Labels and Declarations-Self-declared Environmental
Claims.
For example, a recyclable plastic container using this scheme is
marked with a triangle with three arrows inside of it (see picture on the left),
which enclose a number giving the plastic type as follows:









1. PETE or PET (i.e. polyethylene terepthalate:
termoplastic material used in plastic soft drink and rigid
containers)
2. HDPE (i.e. high density polyethylene: the plastic
commonly used to make milk and water jugs and two liter soda bottle
bases)
3. PVC (i.e. polyvinyl chloride)
4. LDPE (i.e. low density
polyethylene: the plastic used in cellophane wrap, diaper liners, and some
squeeze bottles)
5. PP (i.e. a light, thermoplastic resin used in
packaging, coating, pipes, and tubes)
6. PS (i.e. polystyrene)
7. Others -
Incineration
-
Incineration
The incineration of PVC causes the release of dioxins and other
toxic chemicals.
- Landfill
Landfill of PVC has other environmental
and social impacts. This is due to the not biodegradability of PVC which stays
in place indefinitely; besides, attention should be taken to the fact that PVC
may leach out toxic chemicals and contaminate the soil and water.
There
are some "biodegradable" plastics that break down with exposure to sunlight but
it still doesn't lead to complete breakdown of the plastic. In addition, some
researchers have genetically engineered bacteria that synthesize a completely
biodegradable plastic.
Market applications
Construction material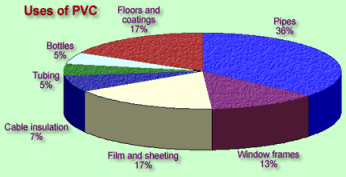
Due to PVC
properties, as described above, around 50% of PVC (or vinyl) manufactured is
used in construction replacing other materials such as wood or glass. Cheap,
resistant, good weatherabiligy, ect.
PVC is strong, lightweight,
durable and versatile. These characteristics make it ideal for window profiles.
PVC's inherent flame retardant and excellent electrical insulation properties
make it ideal for cabling applications.
It can be used for flooring, windows and door
frames and shutters, water and waste pipes, electrical applications such as
cable and wire insulation materials, architectural glazing systems, wallpaper,
etc.
Medical devices
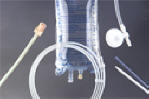 PVC has been
widely used for surgery, pharmaceuticals, drug delivery and medical packaging.
Some products include blood bags, medical containers, fluid bags, tubing, heart
and lung bypass sets, masks, gloves, bottles and jars, drainage systems,
ducting, etc.
PVC has been
widely used for surgery, pharmaceuticals, drug delivery and medical packaging.
Some products include blood bags, medical containers, fluid bags, tubing, heart
and lung bypass sets, masks, gloves, bottles and jars, drainage systems,
ducting, etc.
The reasons to use it in the medical sector is its safety and chemical stability and bio-compatibility, chemical resistant and low cost. In addition, it is usable inside the body and easy to be sterilized.
Automotive
Typical examples of PVC
automotive components include: moldings, interior door panels and pockets, seat
coverings, sun visors, seals, floor covering, wiring, exterior side molding and
protective strips, anti-stone damage protection, etc. Brakes
Other
applications
 PVC can be used for manufacturing toys, packaging,
electric and electronic equipment, household goods, coating, plastic parts in
motor vehicles, office supplies, insulation and
PVC can be used for manufacturing toys, packaging,
electric and electronic equipment, household goods, coating, plastic parts in
motor vehicles, office supplies, insulation and adhesive tapes,
furniture, etc.
adhesive tapes,
furniture, etc.
For consumers in shoe soles, children's toys,
handbags, luggage, seat coverings, etc.
Industrial sectors for conveyor
belts, printing rollers.
Electric and electronic equipment such as circuit
boards, cables, electrical boxes, computer housing.
Material notes
|
|
|---|
|

Copyright (c) 2004 TW Enterprises. All rights reserved.






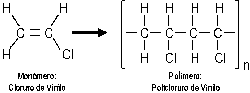
 PVC is a
thermoplastic material.
PVC is a
thermoplastic material. Title
Title